Learning Outcomes:
i. Define oxidation and reduction in the context of organic chemistry.
ii. Identify organic redox reactions based on the concept of electron transfer.
iii. Recognize common types of organic redox reactions, including combustion, halogenation, and dehydrogenation.
iv. Analyze the role of oxidizing and reducing agents in organic redox reactions.
v. Appreciate the significance of organic redox reactions in various biological and industrial processes.
Introduction
Redox reactions, involving the transfer of electrons between species, are ubiquitous in organic chemistry. In this lesson, we will explore the realm of organic redox reactions, delving into the mechanisms, types, and significance of these reactions.
i. Oxidation and Reduction: The Electron Dance
In organic redox reactions, oxidation refers to the loss of electrons, while reduction refers to the gain of electrons. These electron transfers lead to changes in the oxidation state of the atoms involved.
Oxidation: During oxidation, an organic compound loses electrons, resulting in an increase in its oxidation state. For instance, the conversion of ethanol (C2H5OH) to acetaldehyde (CH3CHO) involves the loss of two electrons, raising the oxidation state of the carbon atom from −1 to +1.
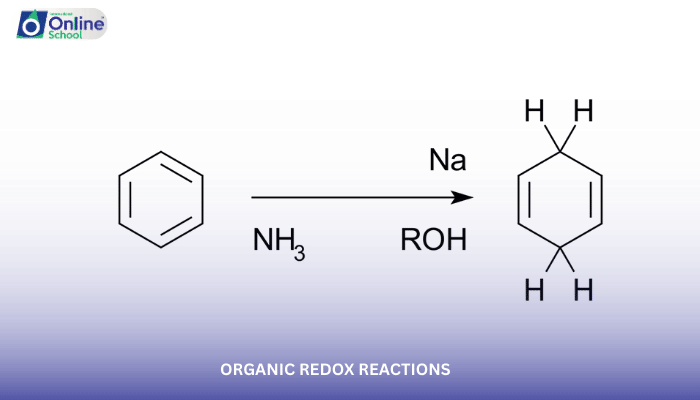
Reduction: Conversely, during reduction, an organic compound gains electrons, leading to a decrease in its oxidation state. For example, the reduction of ethene (C2H4) to ethane (C2H6) involves the gain of two electrons, lowering the oxidation state of the carbon atoms from −2 to −3.
ii. Common Types of Organic Redox Reactions
Organic redox reactions manifest in various forms:
Combustion: The most familiar organic redox reaction is combustion, where an organic compound reacts with oxygen, releasing heat and light. For instance, the combustion of methane (CH4) produces carbon dioxide (CO2) and water (H2O).
Halogenation: The reaction of alkanes with halogens, such as chlorine or bromine, to form haloalkanes involves the oxidation of the alkane and the reduction of the halogen.
Dehydrogenation: The removal of hydrogen atoms from an organic compound, resulting in the formation of a double or triple bond, is another type of organic redox reaction. For example, the dehydrogenation of ethane (C2H6) to produce ethene (C2H4) involves the oxidation of ethane and the reduction of the hydrogen atoms.
iii. Oxidizing and Reducing Agents: The Catalysts of Redox
Oxidizing and reducing agents play crucial roles in organic redox reactions:
Oxidizing Agents: Oxidizing agents accept electrons from other species, causing them to undergo oxidation. Common oxidizing agents in organic chemistry include oxygen, halogens, and metal ions.
Reducing Agents: Reducing agents donate electrons to other species, causing them to undergo reduction. Common reducing agents in organic chemistry include hydrogen, metals, and some organic compounds.
iv. Significance of Organic Redox Reactions
Organic redox reactions are fundamental to various biological and industrial processes:
Cellular Respiration: The energy-generating process in cells, where glucose is oxidized to produce ATP, involves a series of organic redox reactions.
Photosynthesis: The conversion of light energy into chemical energy in plants involves organic redox reactions, where carbon dioxide is reduced to glucose.
Industrial Processes: Organic redox reactions are employed in various industrial applications, including the production of pharmaceuticals, polymers, and synthetic fibers.
Organic redox reactions, driven by the transfer of electrons, encompass a wide range of reactions with profound significance in chemistry and biology. Understanding the principles of oxidation and reduction, the different types of organic redox reactions, and the role of oxidizing and reducing agents is essential for comprehending the reactivity of organic compounds and their applications in various fields.